Authors: Zelin Cui [1]], Sebastian Leptihn [2]
| Article Received: | 18/12/2020 |
| Received: | 20/12/2020 |
| Accepted: | 27/12/2020 |
A recent article “Safety of bacteriophage therapy in severe Staphylococcus aureus infection” published in March 2020 in Nature Microbiology evaluated the safety of phage therapy in phase I clinical trials. The pharmaceutical parameters of the phages in the blood which administered by intravenous injection were monitored. At the beginning of the administration during the initial 5 days, intact phages were observed in blood samples, assessed by bacterial plaque assays. However, in the subsequent 9 days no intact phages were found, while their DNA could be detected by using real-time PCR, posing the question why no intact phage particles are being detected in the later course of administrations. Here, we postulate that antibodies induced during the initial rounds of phage therapy would lead to inactivation the therapeutic virus.
Keywords: Phage therapy; antibody; therapeutic efficacy
Phage therapy has gained renewed attention due to the emergence and spread of multi-drug-resistant bacteria, especially to treat infections caused by pan-drug resistant bacteria. Several successful cases of phage therapy have been reported in recent years including for the infections caused by Klebsiella pneumoniae, Acinetobacter baumannii, Mycobacterium abscessus and others [1,2]. Furthermore, several phase-I clinical trials recently showed that there are no obvious adverse effects during phage therapy, administered intranasal or intravenously [3,4]. In contrast to antibiotics used in the treatment of infectious diseases, bacteriophage are virus containing both, proteins and nucleic acid, and thus could induce the production of neutralizing antibodies even when administered orally and topically or via rectal delivery [5,6]. The report above describes that intact phage particles can not be observed during later stages of therapy, and thus we hypothesize that the patient developed specific anti-phage antibodies, induced in the initial phase of the phage treatment, explaining why only free DNA but not particles are observed. Such antibodies may be able to block the therapeu- tic efficacy in humans.
The recently published article “Safety of bacteriophage therapy in severe Staphylococcus aureus infection” in March 2020 in Nature Microbiology evaluated the safety of phage therapy in phase-I clinical trials [3]. In this study, the dynamic of the phages in the blood were monitored up to four hours after each round of intravenous administration: using the bacterial plaque assay, intact phages remain observable for four hours in blood samples only during the first ten rounds of administration in the initial five days during the entire two week therapy. From day 6-14, no viable phages can be detected despite the fact that identical preparations of the therapeutic phages were administrated in an identical way. While infectious phages decrease to levels below the detection limit, high copies of phage DNA remain detectable by real-time PCR.
The authors of this important study do not speculate on the presence of intact phages during the treatment. We postulate that the patient’s anti-bodies, which are induced during the initial phase of the intravenous administration of therapeutic phages, could possibly bind to the phages and re- duce their therapeutic efficacies. It is well estab- lished for viruses that infect eukaryotic cells, that antibodies have the ability to prevent infection, and mediate the removal of the pathogen from the host system, thus providing protection [6,7]; for example, the antibodies induced by spike protein receptor-binding domain (S-RBD) of SARS-CoV-2 is able to block the virus from entering the host cell 8. In the phage therapy trial by Fabijan AP et al. however, the authors did not assess whether or not phage specific antibodies have been generate by the patient’s immune system3. One study has shown that a phage infecting the pathogen Klebsiella penumoniae phage, administered intravenously in a mice model, induced the production of efficient phage neutralizing antibodies9. Among others, one possible mechanism how the anti-phage antibodies exhibit their neutralising properties is by the blocking and steric obstruction of phage proteins that mediate the binding to the re- ceptor on the bacterial host. This may be one of the reason that high copies phage DNA are detectable during the last 2/3 of the therapy, but no intact phages.
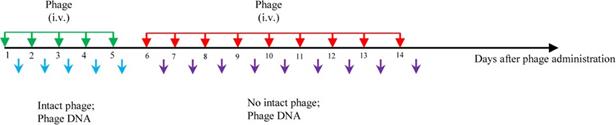
Fig.1 Phage administration strategy and monitor of phage. Phages were administered by intravenous injection twice a day for a total of 14 days. Intact phage in blood were detected using bacterial plaque assays. The phage DNA was detected using real-time PCR. Green arrows indicate the earlier rounds of administration in the initial 5 days, red arrows indicate the later administration in sequential 9 days. Light blue arrows mean that both intact phages and their DNA could be detected. Purple arrows indicate there were no intact phage particles were detected, but high copies their DNA.
In summary, while our conclusion remains speculation, it is important to emphasize that antibodies induced during phage therapy have the ability to block their later efficacies when using the same phages for therapeutic purposes for prolonged periods of time. Further studies are required to conclusively demonstrate that antibodies negatively interfere with phage therapeutic interventions. Should this be the case, administration has to opti- mised to increase the success rates for the treatment, by altering the administered dosage, the frequency and route, but also the choice of phages [10].
Thanks Tingting Feng of Shanghai General Hospital for revision the manuscript.
NIL
The authors declare that there is no conflict of interest.
1. Dedrick, R. M. et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nature Medicine 2019;25: 730-3,
2. Schooley, R. T. et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acineto-bacter baumannii Infection. Antimicrob Agents Chemother 61, doi:10.1128/AAC.00954-17 (2017).
3. Petrovic Fabijan, A. et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol, doi:10.1038/s41564-019-0634-z (2020).
4. Merabishvili, M. et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4, e4944, doi:10.1371/journal.pone.0004944 (2009).
5. Lusiak-Szelachowska, M. et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol 2014;27: 295-304. .
6. Gorski, A. et al. Phage as a modulator of immune responses: practical implications for phage therapy. Adv Virus Res 2012;83:41-71.
7. Lacek, K. et al. Novel human SR-BI antibodies prevent infection and dissemination of HCV in vitro and in humanized mice. J Hepatol 2012;57:17-23. .
8. Yang, J. et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature, doi:10.1038/s41586-020-2599-8 (2020).
9. Songjian, C. et al. Pharmacokinetics of the Podoviridae phage LH-01 in mice infected with Klebsiella pneumoniae Journal of Pathogen Biology 2016;11:616-9.
10. Cui, Z., Guo, X., Feng, T. & Li, L. Exploring the whole standard operating procedure for phage therapy in clinical practice. J Transl Med 2019;17: 373.
Zelin, Sebastian. Anti-phage antibodies induced during phage therapy decrease efficacies of therapeutic phages. Int J Bacteriophage Res 2021:1:35-6.