Authors: VinodKumar C.S [1], Suneeta Kalasuramath [2], Srinivasa H [3], Ananyaa Gopalakrishnan [4], Pallavi Priya [4], Abubakar Sabo Bello [4]
| Article Received: | 28/12/2020 |
| Received: | 06/01/2021 |
| Accepted: | 12/01/2021 |
Context: Almost all MDR/XDR Gram-negative bacteria were resistant to first and second-generation cephalo- sporin, and monocyclic beta-lactam. They were relatively sensitive to meropenem, amikacin, and tigecycline. Emergence of multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria and the limited pro- spects of producing new antibiotics have opened up the second window for the bacteriophages.
Objectives: To predict inoculum size of bacteriophage against carbapenem resistant Klebsiella pneumoniae and to study the kinetics of different dose of host and the bacteriophage by using cell growth assays.
Material & Methods: Two different doses of MRSA (102 CFU/ml and 108 CFU/ml) were challenged with Klebsiella pneumoniae phages (102 PFU/ml and 109PFU/ml) and the bacteriophage kinetics were monitored in vitro by using cell growth assay for 99 hours. Comparison of bacteriophage kinetics was determined by plotting area under the graph for every 3 hours till 18th hour.
Results: When 108 CFU/ml of carbapenem resistant Klebsiella pneumoniae were challenged with 109PFU /ml of Klebsiella pnemoniae phage, the bacteria growth was reduced when compared to normal growth of carbapenem Klebsiella pneumoniae (Bacterial control). Six fold reductions in mean area were observed. Similarly, the reduc- tion of growth was more pronounced when 102 CFU of carbapenem resistant Klebsiella pneumoniae was chal- lenged with 109PFU/ml of Klebsiella pneumoniae phage. Mean area was almost same compared to the phage control at 12th hour indicating the clearance of the bacteria. But when lower concentration of phages (102 PFU/ ml) was used against higher concentration of bacteria, the rate of reduction was not as efficient when compared to higher concentration of phages (109 PFU/ ml).
Conclusion: In dose dependent study when bacteria and phages were in same concentration, it took longer duration for bacteriophage to clear the bacteria. This agrees with the idea that bacteriophage action against host is part of the adaptive response. But when higher concentration of phages was used against lower concentration of the bacteria (initial dose) bacteria were cleared in short duration. Implying for active effective therapy higher concen- tration of phages are required and even lower concentration of phages could give rise to passive effective therapy.
Keywords: Bacteriophage, kinetics, dose dependent study, carbapenem resistant Klebsiella pneumoniae
Increase of resistance among gram-negative bacteria are gradually on the rise and it appears that control measures may not yet be successfully accomplished. Multi-drug resistant bacteria often complicate treatment options and result in unfavourable morbidity. New resistance mechanisms are emerging and spreading globally, threatening our ability to treat common infectious diseases [1,2]. Ironically, resistance is promoted by both the overuse of antibiotics as well as insufficiency of dose. In industri- alized countries, bacteria are developing multiple resistance to a range of antibiotics, which threatens to make the achievements of modern medicine futile [3,4]. In developing countries basic medical care is already endangered by single resistance to inexpen- sive common generic antibiotics [2,5], particularly be- cause of the concomitant increase in immunosuppressed patients [5].
The rapid spread of ESBL with special reference to CTX-M which is highly endemic in developing countries indicates a more difficult situation to control ESBL producers which are now recognized as a cause of community-onset infection. In addition, probably due to a overuse of carbapenem molecule against ESBL producers has led to emergence of high incidence of carbapenem-resistant Gram negative bacilli among family of Enterobacteriaceae [3,4].
Antibiotic resistance is accelerated by the misuse and overuse of antibiotics, as well as poor infection prevention and control. Without urgent action, we are heading for a post-antibiotic era, in which common infections and minor injuries can once again kill. The concern that humankind is re-entering the pre-antibiotic era has become very rea [l1], and the development of alternative anti-infection modalities has become one of the highest priorities of modern medicine and biotechnology. One such substitute is the possible therapeutic use of bacteriophages viruses that parasitize and kill bacteria6. The suggestion of administer- ing phages as alternative to antibiotics has been proposed for more than 100 years, and every so often is hyped by the media7 as a possible “magic bullet.” Studies of bacteriophage therapy have had a history of being rather inadvertent and inci- dental, phages that actively replicating and lyse bacteria in vitro do not always do so invivo6-9. The poor predictability of outcome has been attributed to a various causes; host contamination, phage bacteria specificity, dose variability, anti- phage host immune response [10,11], bacterial co-evolution /adaptability to the phages and horizontal toxin transfer via temperatephages [12,13]. The main objectives of the present study to predict inoculum size of bacteriophage against Cr-KPN and to study the kinetics at different dose of host and the bacteriophage by using cell growth assays.
The research protocol and the consent procedures were approved by the Institutional Ethics Review Board of St. Johns Medical College, Bangalore and S. S. Institute of Medical Sciences and Research Centre, Davangere, Karnataka since study was conducted at both the institutes.
Klebsiella pneumoniae strains were isolated from pus of a diabetic foot [4]. Antibiotic susceptibility testing by Kirby-Bauer’s method revealed that the Klebsiella pneumoniae isolated was multidrug resistant bacteria. The Klebsiella pneumoniae strain was resistant to carbapenem. Resistance to carbapenem is mediated by two most common mechanisms. First, Klebsiella pneumoniae is able to produce β-lactamases with the ability to hydrolyze cephalosporins such Extended spectrum beta lactamases (ESBL) or AmpC cephalosporinase [2,3]. The second mechanism is mediated by the production of a β-lactamases capable of hydrolyzing most β-lactams antibiotics including carbapenems.
Bacterial inoculum was prepared by inoculating Cr-KPN in nutrient broth, incubating at 37oC overnight followed by repeated centrifugation (10,000rpm for 10 mins) and washing, finally re-suspending in normal saline.
The phages were isolated from different sources of water by the method of Smith and Huggins. Sewage6,7 water (50ml) was collected in sterile conical flask and treated with a few drops of chloroform. To this 5ml of lactic phage broth and 1 ml of the 24 hrs old broth cultures were added. The sample inoculated with Cr-KPN was incu- bated at 37oC for 12-24hr in shaker water bath. After 12-24hrs the lysate was shaken with few drops of chloroform for about 10 min, centrifuged at 10,000 rpm for 10 min and the supernatant was filtered through 0.22m pore size Acrodisc membrane filters (PALL, German Laboratory) to remove the bacteria and subjected to plaque forming unit (PFU) assay using double layer agar method described by Smith and Huggins [6]. Phage preparations to be used therapeutically were passed through a column containing Detoxi-Endotoxin Removing Gel (Pierce, Rockford, IL) as recommended by the manufacturer (Pierce instructions http://www.piercenet.com) and eluted with pyrogen-freewater.
The bacterial lawn was prepared on nutrient agar plates employing 1.0ml of 24hr culture by flooding and draining out the excess. Wells were dug into the agar by employing a sterile cork borer and the 20 ml phage suspension were loaded into each of the well. Sterile distilled water served as the control. The plates were incubated at 370C for 24 hr. There after the zone of inhibition, if any, was recorded [16,17].
The plaques if obtained were further passaged on the same target bacterial host to reconfirm its activity.
Quantifying analysis performed on the basis of extinction co-efficient, without standard curve. When using a cuvette, the path length is known and is independent of sample volume, so absorbance is proportional to concentration. Where else in a microplate, path length is dependent on the liquid volume, so absorbance is proportional to both the concentration and the path length of the sample. In a kinetic read the data are collected over time with multiple reading taken at regular intervals. The kinetic analysis can be performed up to 99 hours.
Kinetic analysis is capable of providing improved dynamic range, precision and sensitivity relative to endpoint analysis
Bacteriophage experiments were performed at 370C in microplate containing 200µl of culture using a spectra max plus micro titer plate reader with softmax pro software (Molecular devices). The turbidity at 650 nm was measured every 10 minutes and plate was shaken for a period of 30 seconds before each measurements. The detailed protocol is given in the table-1.
Various methods are available for determination of area under the curve. The four recognized methods commonly used are; use of planimeter, counting squares, trapezoidal rule and cutting and weighing. In the present study we have used counting square method to calculate the area under the different optical density verses time curve. As the name suggests the total number of squares enclosed by the bacterial growth or phage growth verses time curve are counted [21,22]
The time duration and optical density were plotted on X and Y axis in 1mm2 graph sheet, from which area under the graph was calculated for every 3 hours till 18th hour. The optical density as a function of time is plotted on a regular rectilinear graph paper and instead of joining the points with straight lines, a smooth curve is drawn to best represent the data point. No attempt is made to extrapolate the curve beyond the last OD time point. Instead, a straight line is drawn to connect the last concentration data point with corresponding time point on the time axis. The squares enclosed within this bounded growth curve verses time curve are now counted. The area of each square is determined using the relationship: area= (height) (width) at the desired time interval.
Count of bacteriophage were obtained and expressed in term of plaque forming unit and then converted to logarithms to the base 10. ANOVA was used to compare within the group and among the groups.
Out of 300 diabetic cases included in the study, 61 pus samples from diabetic foot yielded growth for Klebsiella pneumoniae. All 61 strains of Klebsiella pneumoniae were resistant to two or more antibiotics and screening for drug resistance mechanisms revealed 47 strains were extended spectrum beta lactamse producers and 12 strains were metallo beta lactamase producers.
Phage against Cr-KPN was isolated from lake water, sewage water and open drainage water. The electron microscopy of the phages isolated against Cr-KPN had an icosahedral head, measuring about 65-100 nm in diameter, and a 100-120nm long tail. Based on the morphology and the rules provided by International Committee on Taxonomy of Viruses (ICTV, Bethesda MD, USA) the phage was tentatively placed in the Siphoviridae family.
The Antibacterial activity of phage against Cr-KPN revealed that the phage was found to form plaques on 95% of the Cr-KPN isolates cultured from diabetic foot infection. The inhibition of bacterial growth in strains that the phage could not form plaques is most likely due to partial expression of the phage genome, sufficient for killing but not enough for phage production to a level necessary for plaque formation.
Host specificity testing revealed that phage lysed all Klebsiella pneumoniae strains tested. Within this lytic spectrum, clear plaques were produced on all strains except on few strains of Cr-KPN which had a mixture of the opaque and clear plaques. Plaque size of phages from different source ranged from 1.0 mm to 9.0 mm. Klebsiella pneumoniae phage lysed all Klebsiella pneumoniae, and showed lesser activity against other species of Klebsiella. But, no plaques were seen when Klebsiella pneumoniae phage were treated with Pseudomonas aeruginosa or Acinetobacter baumannii.
| Well | Descriptions | Quantity |
|---|---|---|
| A1 | Blank 1 | 200µl of distilled water |
| B1 | Blank 2 | 200µl of Brain Heart Infusion broth |
| C1 | Blank 3 | 200µl of Lytic Phage broth |
| D1 | Bacterial control 1 | 200µl of 108CFU of CR-KPN |
| E1 | Bacterial control 2 | 200µl of 102CFU of CR-KPN |
| F1 | Phage control 1 | 200µl of 109 PFU of Klebsiella pneumoniae phage |
| G1 | Phage control 2 | 200µl of 102 PFU of Klebsiella pneumoniae phage |
| A2 | Test 1 | 100 µl of 108CFU of CR-KPN is challenged with 100µl of 109 PFU Klebsiella pneumoniae phage |
| B2 | Test 2 | 100 µl of 108CFU of CR-KPN is challenged with 100 µl of 102 PFU of Klebsiella pneumoniae phage |
| C2 | Test 3 | 100 µl of 102CFU of CR-KPN is challenged with 100 µl of 109 PFU of Klebsiella pneumoniae phage |
| D2 | Test 4 | 100 µl of 102CFU of CR-KPN is challenged with 100 µl of 102 PFU of Klebsiella pneumoniae phage |
| D3 | Test 5 | 100 µl of 102CFU of CR-KPN is challenged with 100 µl of Piperacillin 32µg |
Table: 1: Protocol for Bacteriophage kinetics of Klebsiella pneumoniae phage by Cell growth assays by using Spectramax 340.
Bacteriophage kinetics of Klebsiella pneumoniae phage at different concentration against Cr-KPN at different concentration at different time inter- vals is depicted in table 2 to 5. The bacteria in the lag phase were taken for the experiments. In the bacterial control (108 CFU), initially there was increase in the optical density (OD) values till 250 minutes (Table 1). After 630 minutes to 810 minutes of incubation, there was no appreciable difference in the OD (stationary phase), after 810 minutes till the end of the experiment the OD values started dropping (decline phase) significantly (P0.001). Similar for the bacteria of concentration 102 CFU, increase in the OD values was ob-served till 130 minutes of incubation (Table 3), later there were no appreciable differences in the OD values till 680 minutes (Stationary phase). After 680 minutes of incubation, the OD values started dropping which was statistically signifi-cant (P0.001).
In dose dependent study, Klebsiella pneumoniae phage was more effective when the host bacteria and the phages were in the same concentration. When the bacteria of the concentration of 108 CFU were challenged with bacteriophage of the concentration of 109 PFU, the mean area under the curve increased during 3rd of incubation and decreased during 6th, 9th, 12th and 15th hours (Table-6) (ANOVA, P0.001). Approximately three fold decrease in the mean area was observed between 3rd hours to 18th hours. But when the bacteria of concentration of 102 CFU were challenged with bacteriophage of the con- centration of 109 PFU, the bacterial load started decreasing sharply at the 3rd hour after challenging with the phages and the decrease was more efficient at 18th hour when compared with standard bacterial control and the antibiotic challenged group (Table-7) (ANOVA, P0.001). No bacteria were recovered at 15th hour after challenging with Klebsiella pneumoniae phage. The OD value at 15th hour was same as the OD value of bac- teriophage control. Where else when the bacterial concentration of 108 CFU were challenged with bacteriophage of the concentration of 102 PFU, the mean area under the curve increased during first one hour but later started decreasing at 3rd and 6th hour of incubation and steady decrease was observed during 9th, 12th and 15th hours (Table-8, Fig.2) (ANOVA, P0.001). For the bacteria of the dose 102 CFU were challenged with bacteriophage of the concentration of 102 PFU, steady decrease in the mean area was observed from 3rd hour to 15th hour (ANOVA, P0.001) (Table-5 & 8, Fig 1).
Turbidity growth curve at 370C were generated for piperacillin against Cr-KPN using the Spectramax instrument. Growth was followed by measuring the turbidity every 10 minutes for up to 18 hours in wells inoculated with 100 µl of bacterial suspensions of an initial concentration of approximately 108 CFU and 100 µl piperacillin of different concentration. The microplates were shaken for 30 seconds prior to measurement of turbidity. triplicate wells in three replicate experiments.
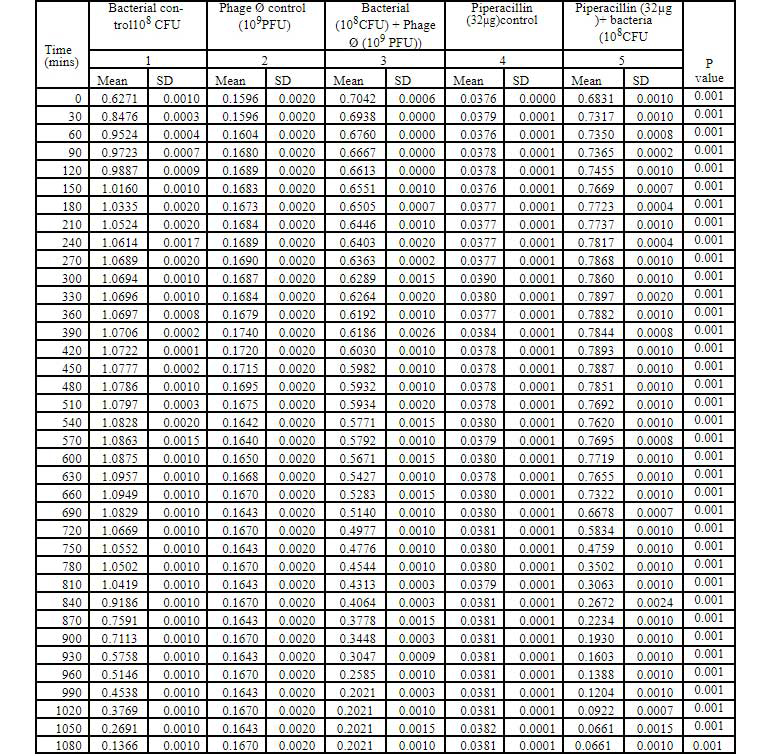
Table 2: Bacteriophage kinetics of klebsiella pnemoniae phage (10PFU) against Cr-KPN (10CFU) at different time intervals.
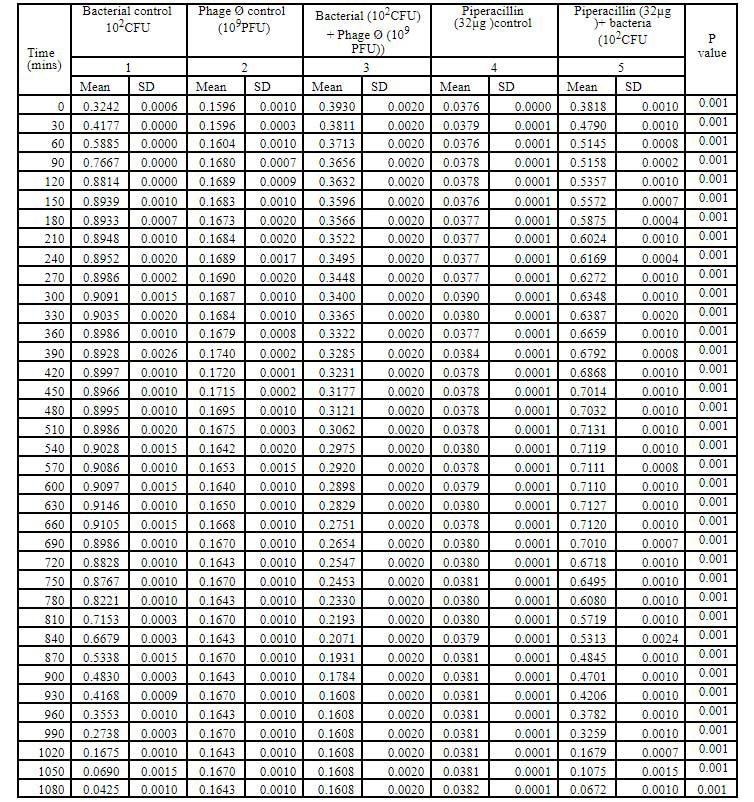
Table 3: Bacteriophage kinetics of klebsiella pnemoniae phage (10PFU) against Cr-KPN (10CFU) at different time intervals.
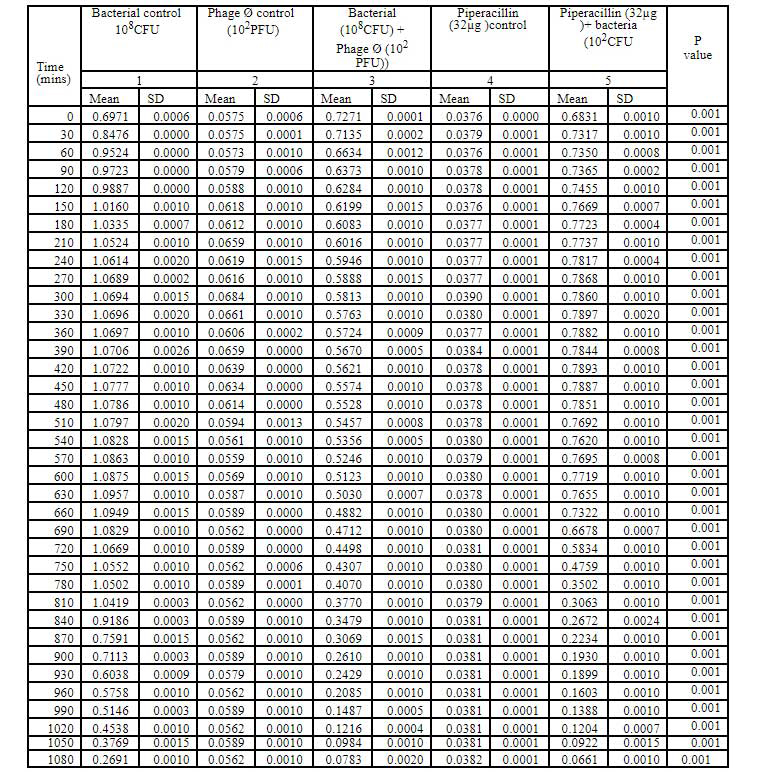
Table 4: Bacteriophage kinetics of klebsiella pnemoniae phage (10PFU) against Cr-KPN (10CFU) at different time intervals.
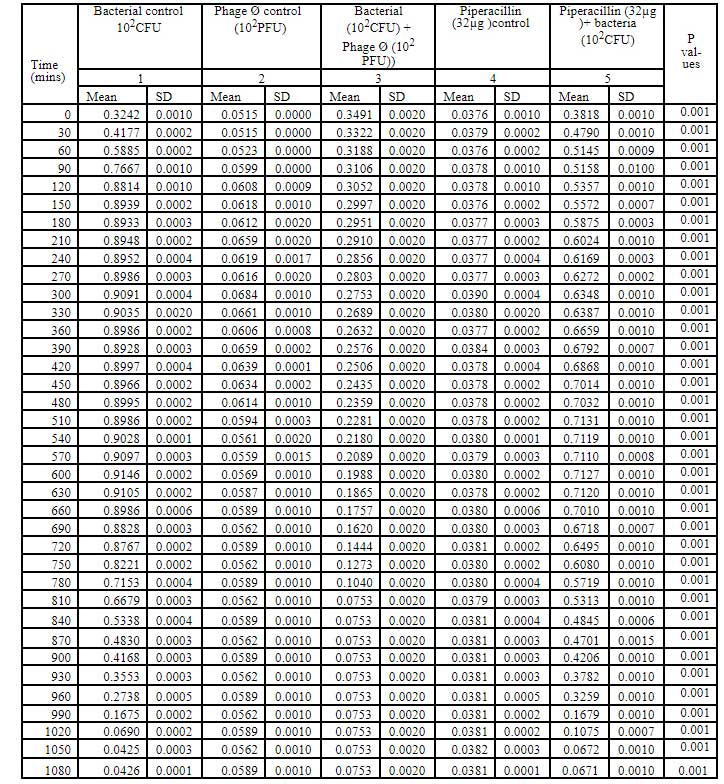
Table 5: Bacteriophage kinetics of klebsiella pnemoniae phage (10PFU) against Cr-KPN (10CFU) at different time intervals.
The break point for piperacillin for Klebsiella pneumoniae is 16µg. In the present study the MIC for piperacillin was 32µg was used. The growth curve generated showed steady decrease in the mean area from 3rd hour to 18th hour for different concentration of bacteria. Mean area was larger for lower concentration compared to higher concentration of piperacillin. The mean area un- der the curve for piperacillin of concentration (32µg) and bacterial density (108 CFU) was larger when compared to phage of concentration 109 PFU till 12th hour (Fig-1,2) (ANOVA, P0.001). Even though the steady decrease in the mean area for the piperacillin of concentration 32µg to the bacterial density 102 CFU was observed, but it was not as effective as the phage at the concentration of 109 PFU for 108 CFU (Table-2, Fig 2) or for the phage concentration of 102 PFU to 102 CFU.
Since the dawn of the antimicrobial drug era, resistance has shadowed the success of infectious disease therapy. In his 1945 Nobel prize acceptance speech, Alexander Fleming noted the danger of resistance: “ It is not difficult to make microbes resistance to penicillin in the laboratory by exposing them to concentration not sufficient to kill them and same thing has occasionally happened in the body. MRSA are now resistant to essentially all available antimicrobial drugs and some remain susceptible to few. At the same time what once was an apparent deluge of antimicrobial drug development is now barely a trickle. The lack of new drug classes is a consequence of difficulties in discovery of new compounds that has persisted for many years.
Barring the arrival in the near future of new antimicrobial drugs that are effective against disparate organisms, we are left with imperfect tools to control drug resistance, with notable exception [2,3]. Infection control in healthcare settings, which is essential for preventing transmission of susceptible and resistant microorganisms alike, remains imperfect. Reducing the discretionary use of antimicrobial drug when possible is helpful but even if we use these drugs with exquisite precision, resistance will continue to evolve and spread [4,5]. Hence the development of alternative antiinfection modalities has become one of the highest priorities of modern medicine. One of such alternatives stems up from an old idea is the bacteriophage therapy. Although phages were discovered nearly a century ago, Western medicine’s interest in them as therapeutic agents was relative- ly short-lived, in part because of the eventual discovery and immediate success of antibiotics and in part because of the highly empirical and counter productive approach that had been used by phage practitioners in the early era. In the modern era (1980s and 1990s), some rigorously controlled animal experiments have been conducted by Smith6, but the clinical reports in this same era have been an anecdotal nature rather than describing controlled studies [7,9,12]. Recently phages are used in various veterinary infections, for the treatment of Enterococci infection11 and also in the treatment of MDR Pseudomonas infection [18].
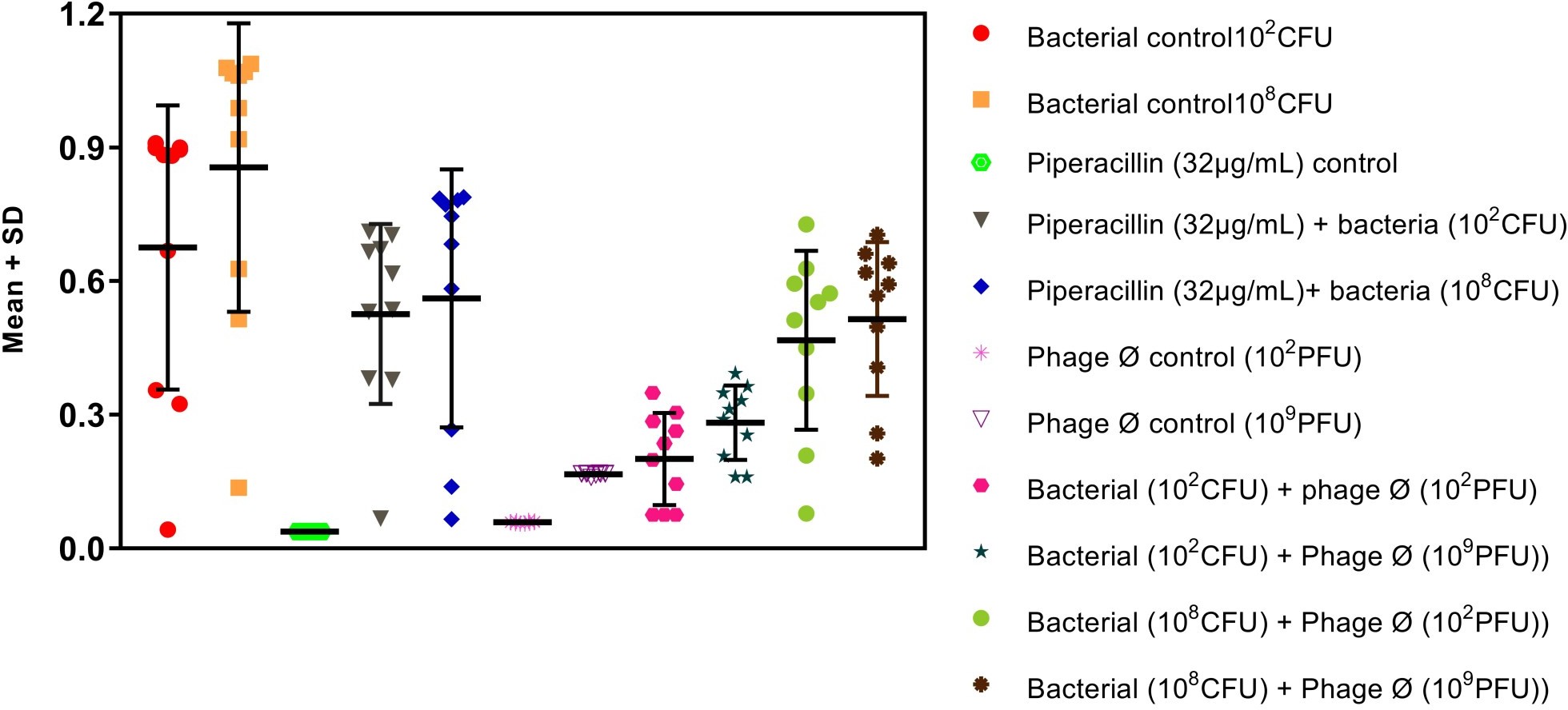
Figure-1: Scatter plot (mean±standard deviation) shows kinetic parameters for bacterial time-kill assay by bacteriophage with different dose.
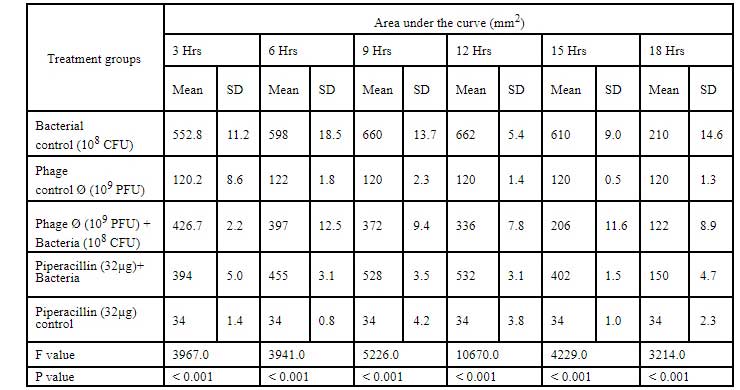
Table 6: Area under the curve for Cr-KPN (10CFU) challenged with klebsiella bacteriophage of 10PFU concentration.
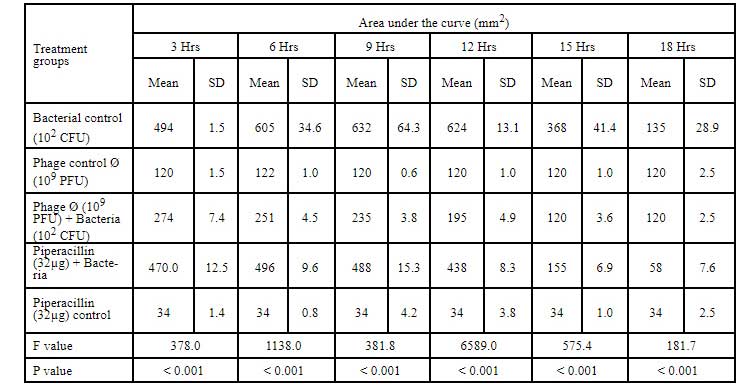
Table 7: Area under the curve for Cr-KPN (10CFU) challenged with klebsiella bacteriophage of 10PFU concentration.
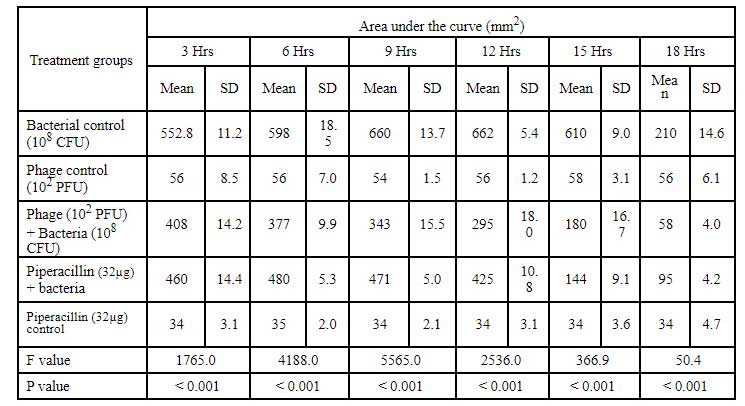
Table 8: Area under the curve for Cr-KPN (10CFU) challenged with klebsiella bacteriophage of 10PFU concentration.
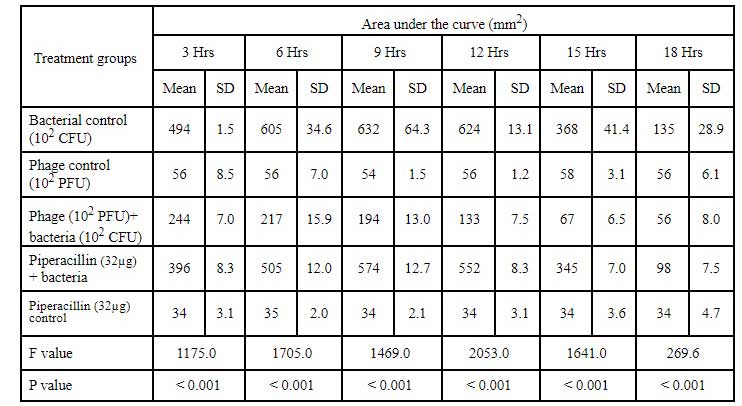
Table 9: Area under the curve for Cr-KPN (10CFU) challenged with klebsiella bacteriophage of 10PFU concentration.
From 1912 to 1940s, hundreds of papers were published describing the use of bacteriophages for the treatment of dysentery and other human infections and commercial companies preparations. But results were mixed, due in large part to a poor understanding of phage biology. Phage preparations were used against bacteria insensitive to that particular phage or even against disease that were not caused by bacteria. In some cases, to prevent bacterial contamination manufacturers added oxidising agents to phage preparations that inactivated the phage. Enthusiasm for the phage treatments soon flagged in the west. Everything that could have been done wrong was done wrong during the first window of opportunity. These and other early errors coupled with the anecdotal nature of clinical research at that time and the discovery of chemical antibiotics led to the rejection of phage therapy by most western doctors by the end of the 1940s. Although the utility of bacteriophages as an alternative for treating bacterial infections was undisputed [19-21], one of the most important factors that interfered with the documentation of the efficacy of phage therapy was lack of scientifically conducted placebo controlled studies. Failures were very common during the early history of phage therapy and therefore the results were frequently controversial, because of paucity of understanding of the heterogeneity of both phages and bacteria. Third problem was failure to select highly virulent phages against target bacte- ria before using them on patients. Use of single phages in infections involving mixtures of different bacteria, was another reason for earlier controversial results. Similar to antibiotics, emergence of resistant bacterial strain by selection of resistant mutants (a frequent occurrence if only single phage is used against a particular bacteria or by lysogenization) was another problem seen in earlier studies [12,13,19]. Failure to appropriately characterize or titre phage preparation, some of which are totally inactive. Failure to neutralize gastric pH before oral administration of phages, inactivation of phages by both specific and non specific factors in body fluids, liberation of endotoxins due to wide spread lyses of bacteria resulting in Jarish-Herxheimer reaction which in turn produced toxic shock, and lack of availability of laboratories which can carefully identify the specific pathogen which is necessitated by the relative specificity of phage therapy.
The experiments in the present study represent elucidations to many of the problems that hindered the prior applications of phage therapy. For example, the relatively narrow host range of most phages which caused many of the early attempts at phage therapy to fail can be trounced by isolating phages that have a broad host range within the species being targeted. For example, The Anti-bacterial activity of Klebsiella phage against MDR Klebsiella pneumoniae revealed that the phage was found to form plaques on 100% of MDR strains and 95% of the Cr-KPN isolates cultured from diabetic foot infection. The bacterial host range of phage is generally narrower than that found in the antibiotics that have been selected for clinical applications. Most phages are specific for one species of bacteria and many are only able to lyse specific strains within a species. This limited host range can be advantageous, in principle, as phage therapy results in less harm to the normal body flora and ecology than commonly used antibiotics, which often disrupt the normal gastrointestinal flora and result in opportunistic secondary infections by organisms such as Clostridium difficile. In the present study, we could demonstrate that Klebsiella phage could form plaques only on the bacteria of the same genus but not on the other genus. The potential clinical disadvantages associated with the narrow host range of most phage strains is addressed through the development of a large collection of well-characterized phage for a broad range of pathogens, and methods to rapidly determine which of the phage strains in the collection will be effective for any given infection.
The issues associated with bacteriophage manufacturing for clinical use include the removal of endotoxins and pyogens released during phage induced lysis and the development of stable formations. Concurrent with the advancement of biotechnology, phage manufacturing has increased in sophistication is capable of producing clinical grade bacteriophage preparations. In our study phage preparations to be used therapeutically were passed through a column containing Detoxi- Endotoxin Removing Gel (Pierce, Rockford, IL) as recommended by the manufacturer (Pierce instructions http://www.piercenet.com) and eluted with pyrogen-free water. This protocol was sufficient to achieve maximum purity for use in a European clinical trial. The pharmacokinetics and pharmaco-dynamics are fundamentally interrelat- ed because phages spread throughout bacterial populations much like epidemics spreading through macro-biological populations: infecting susceptible bacterial cells, reproducing and subsequently infecting other susceptible cells. With respect to phages used against pathogens in clinical condition, one faces a completely different set of premises.
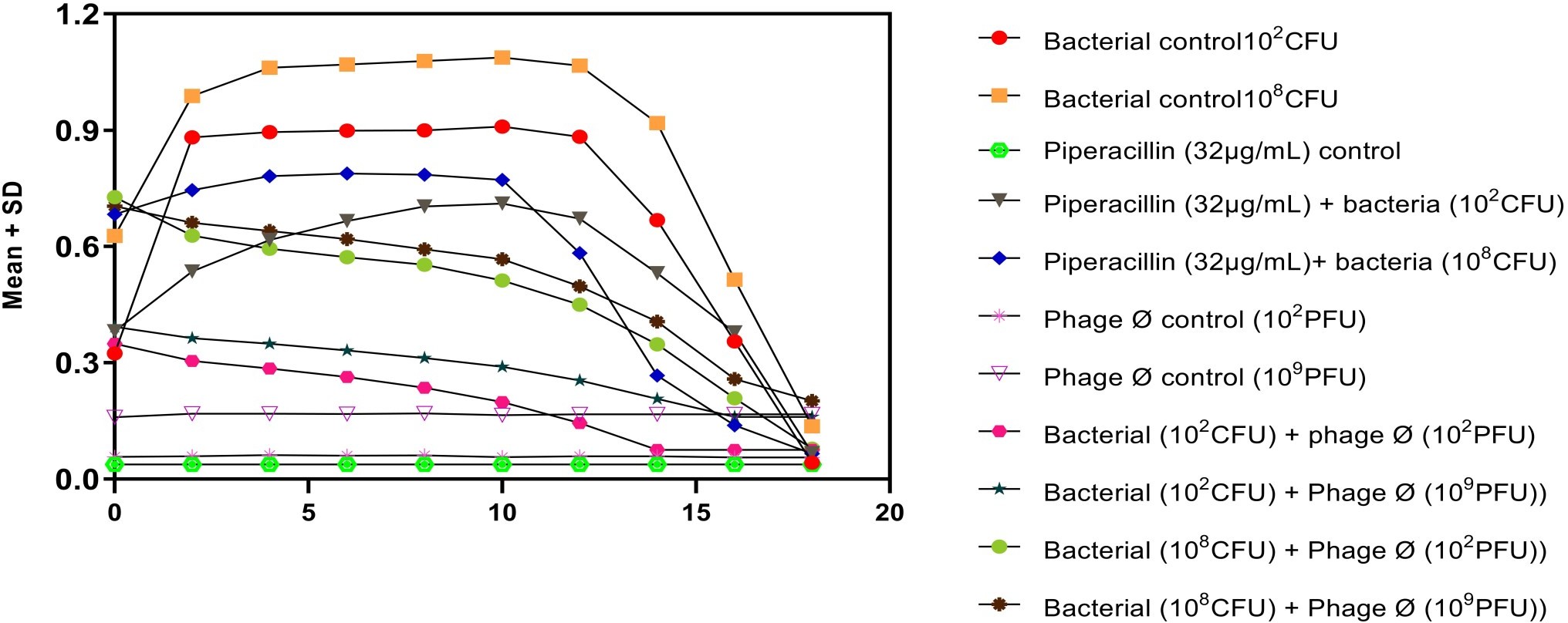
Figure-2: Dose dependent Kinetics of Klebsiella bacteriophages against Cr-KPN
This occurs because a significant proportion to be treated will be solid rather than liquid, and, with modern hygiene regimens in place, any bacterial contamination is likely to occur at very low numbers. Under these circumstances, it is critical to understand that a sufficiently high number of phages are required to hit and infect the few bacterial target cells present. In other words, low numbers of bacteria are unlikely to be affected by low numbers of phages because phages and bacteria are unlikely to meet in the clinical condition. In a more biochemical sense, the concentration of one of the reaction partners (phage) must be sufficiently high to enable contact and subsequent reaction (infection and killing), even when the other reaction partner is present at a very low concentration only (numbers of bacteria). In fact, once a critical concentration threshold of phage numbers is reached to enable it to cover the entire available space within any given wound, the concentration of the bacterial host is not important, i.e., it does not matter whether only 1 or 108 cells per ml are present, they will all be infected.
When 108 CFU of Cr-KPN were challenged with 109 PFU of Klebsiella pneumoniae phage the bacteria growth was reduced when compared to normal growth of MDR Klebsiella pneumoniae (Bacterial control). Three fold reductions in mean area were observed. Similarly the reduction of growth was more pronounced when 102 CFU of Cr-KPN was challenged with 109 PFU of Klebsiella pneumoniae phage Ø. Mean area was almost same compared to the phage control at 15th hour indicating the clearance of the bacteria. But when lower concentration of phages (102 PFU) was used against higher concentration of bacteria, the rate of reduction was not as efficient when compared to higher concentration of phages (109 PFU) [Fig 2]. The growth pattern of host challenged with the drug piperacillin. As per the study the MIC of piperacillin for Cr-KPN was 32 µg (CLSI, break point for Klebsiella pneumoniae is 16 µg). Correlation of the efficacy of phages with the piperacillin revealed that the phage was more efficient during 1st nine hours of incubation, implicating phage kinetics were more efficient compared to piperacillin in- vitro (Fig-2 & Table-6,7)
Wiggins and Alexande22-24 investigated the role of bacterial concentration and found that bacterial concentration of around 104colony-forming units (CFU) mL were required for phage growth on a range of bacterial hosts. But in our study, when 102 CFU of bacteria were challenged with 102 PFU of phages, the bacteria growth was inhibited more efficiently.
To conclude, the concept of a self-replicating, self-regulating antimicrobial that can penetrate into the most corners of the body and selectively combat pathogens is exciting. From our study we elucidated that the when bacteria and phages were in same concentration, it took longer duration for bacteriophage to clear the bacteria. This agrees with the idea that bacteriophage action against host is part of the adaptive response. But when higher concentration of phages was used against lower concentration of the bacteria (initial dose) bacteria were cleared in short duration. Implying for active effective therapy higher concentration of phages are required and even lower concentration of phages could give rise to passive effec- tive therapy.
Authors would like to thank St John’s Medical College and S. S. Institute of Medical Sciences and Research Centre for the facilities and Rajiv Gandhi University of Health Sciences, Bangalore for the permission to carry out the research work.
NIL
The authors declare that there is no conflict of interest.
1. Tanushree S, Pallavi K. Emergence of antibiotic resistance in bacteria. MedDocs ebooks 2019;3 :1-7
2. Boyle DP, Zembower TR. Epidemiology and manage- ment of emerging drug-resistant Gram-negative bacteria: extended-spectrum β-lactamases and beyond. Urol Clin North Am. 2015;42(4):493–505.
3. Cantón R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9(5):466–75.
4. Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18(5):294–8.
5. Nordmann P, Poirel L. Emerging carbapenemases in Gram negative aerobes. Clin Microbiol Infect 2002;8:321–331.
6. Smith HW, Huggins MB, Shaw KM. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophage. J Gen Microbiol 1987;133:1111–1126.
7. Levin BR, Bull JJ Population and evolutionary dynamics of phage therapy. Nature Reviews Microbiolo- gy 2004;2:166–173.
8. Barrow PA. Review; The use of bacteriophages for treatment and prevention of bacterial disease in animals and animal models of human infection. J Chem Technol Biotechnol 2001;76:677–682.
9. Humphreys GO, Trautner TA Maturation of bacteriophage SPP1 DNA: limited precision in the sizing of mature bacteriophage genomes. J Virol 1981;37: 832– 835.
10. Young RY. Bacteriophage lysis: mechanism and regulation. Microbial Rev. 1992;56:430–481.
11. Lenski RE, Levin BR. Constraints on the co-evolution of bacteria and virulent phage: some experiments, and predictions for natural communities. AM Nat 1985;125:585–602
12. Van Helvoort T. Bacteriological and physiological re- search styles in the early controversy on the nature of the bacteriophage phenomenon. Med Hist. 1992;3:243– 270.
13. Bohannan BJM, Lenski RE. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol Lett 2000;3:362.
14. Bauer A W, Kirby W M M, Sherris JC, Jurek M.1966. Antibiotic susceptibility testing by a standardized disc method. American Journal Clinical Pathology 45:493- 496.
15. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; CLSI document M100-S25: CLSI, 2019.
16. Westwater C, Kasman LM, Schofield DA, Werner PA, Dolan JW, Schmidt GM, Noris JS. Use of genetically engineered phage to deliver antimicrobial agents to bacteria: an alternative therapy for treatment of bacterial infections. Antimicrob Agents Chemother 2003;47:1301–1307.
17. Soothill JS. Bacteriophage prevents destruction of skin grafts by Klebsiella sps. Burns 1994;20:209-211.
18. Slopek S, Kucharewicz-Kmkowska, Weber-Dabrowska B, M Dabrowski. Results of bacteriophage treatment of suppurative infections. IV. Evaluation of the results obtained in 370 cases. Arch Immunol Ther Exp (Warsz) 1985;33 (2): 219-240.
19. Slopek S B Weber-Dabrowska B, Dabrowski M, Ku-charewicz-Kmkowska A. Results of bacteriophage treatment of suppurative infections in the years 1981-1986. Arch hnmunol Ther Exp (Warsz) 1987;35: 569- 583.
20. Gowri Shakar R, Madhusudhan V, Palaniappan P. Evaluation of phage therapy to treat experimental infection in mice. Indian Journal of Microbiology 1998;38:101-103.
21. Vinod Kumar. C. S., Srinivasa H, K. G. Basavarajappa and Suneeta Kalasuramath. bacteriophage kinetics: a dose dependent study of methicillin resistant staphylococcus aureus phage. World Journal Of Pharmacy And Pharmaceutical Sciences. 2020:9;5, 1371-1389
22. VinodKumar C.S., Srinivasa H, Basavarajappa K.G, Puttaswamy C.T, Vyshak A, Suneeta Kalasuramath, Bacteriophage as an alternative to chlorine in sewage treatment plant to disinfect multidrug resistant bacteria present in hospital wastewater. Asian Jr. of Microbiol. Biotech. Env. Sc. Vol. 21, No. (2) : 2019 : 246-255
23. VinodKumar C. S, Srinivasa H, Basavarajappa K.G, Umakanth Patil, Nitin Bandekar, Rajashri Patil. Abrogation of Staphylococcus aureus wound infection by bacteriophage in diabetic rats. International journal of pharmacy and drug research. 2011:3(3); 202-207
24. Oi K, Komori H, Kajimura H. Changes in plasma glu- cose, insulin, glucagon, cathecolmine, and glycogen contents in tissue during development of alloxan diabetes in rats. Biochem Mol Med 1997; 62: 70-5.
25. Wiggins BA, Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl. Environ. Microbiol. 1985;49:19-23
VinodKumar C.S, Suneeta K, Srinivasa H, Ananyaa G, Pallavi P, Abubakar S. Modelling inoculum dose dependent kinetics of bacteriophage against carbapenem resistant Klebsiella pneumoniae. Int J Bacteriophage Res 2021:1:21-34.